Physics and mathematics provide insights into growth of new blood vessels
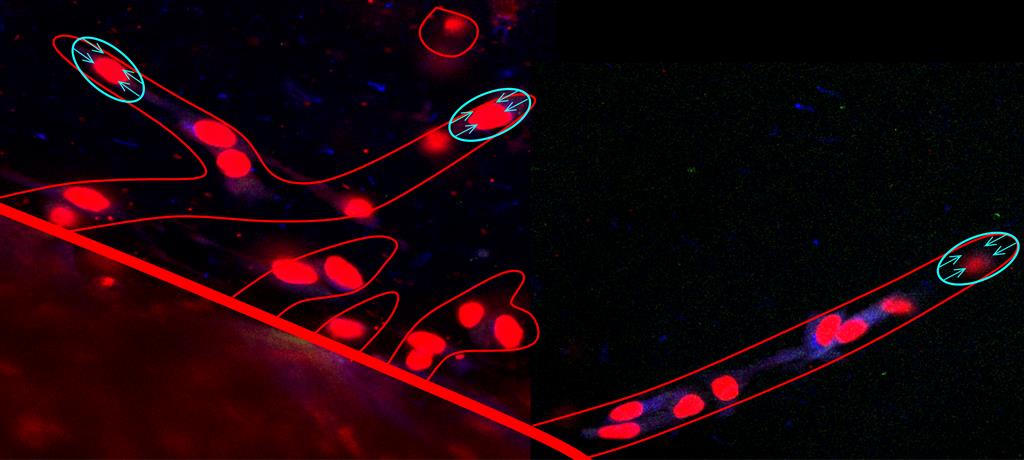
When new organs are formed during development of an embryo, when a wound is healing or during an inflammatory reaction, new blood vessels sprout from existing vessels. These new blood vessels supply cells and tissues with oxygen and nutrients that they need to survive. This process, known as sprouting angiogenesis, also plays a crucial role in the onset and progression of more than 50 different diseases, including cancer, rheumatoid arthritis and diabetes. Indeed, in many cancers, angiogenesis is a fundamental step in the transition from benign to malignant tumors. Understanding this process and designing angiogenesis inhibitors has thus become an important field in cancer research and therapy.
Now, a team of scientists from the University of Coimbra, in Portugal, and the University of Granada (Spain), have used physics and mathematics to unravel the mechanism that underlies how the cells in vessel sprouts move, proliferate and rearrange. Their findings open the door to a new approach to blocking unwanted growth of new blood vessels, one which involves making changes to the physical properties of these cells and neighbouring tissues.
By simulating the growth of new vessel sprouts under different physical and chemical environments (namely the concentration of the Vascular Endothelial Growth Factor – VEGF), the researchers concluded that in the sprouts the cell at the tip creates a tension that spreads to the cells that follow. This tension produces strain or empty spaces behind the lead cell, which triggers the proliferation of new cells. The new cells occupy the space behind the tip, the tension decreases, and the process restarts.
Rui Travasso, a physicist at the University of Coimbra who led this research, highlights the interdisciplinary nature of the team, which brought together physicists, biomedical engineers, clinicians and biologists, “Our computational model comes from experiments carried out by researchers in the University of Coimbra Medical School, and is the first to show how the proliferation of cells in the new vessels seems to depend on the mechanical tension that the new vessel is under during growth, as well as on VEGF concentration, for functional blood vessels to form.”
This study was published in the open-access journal PLoS Computational Biology, and featured on the issue cover.
(Image credits: University of Coimbra)